
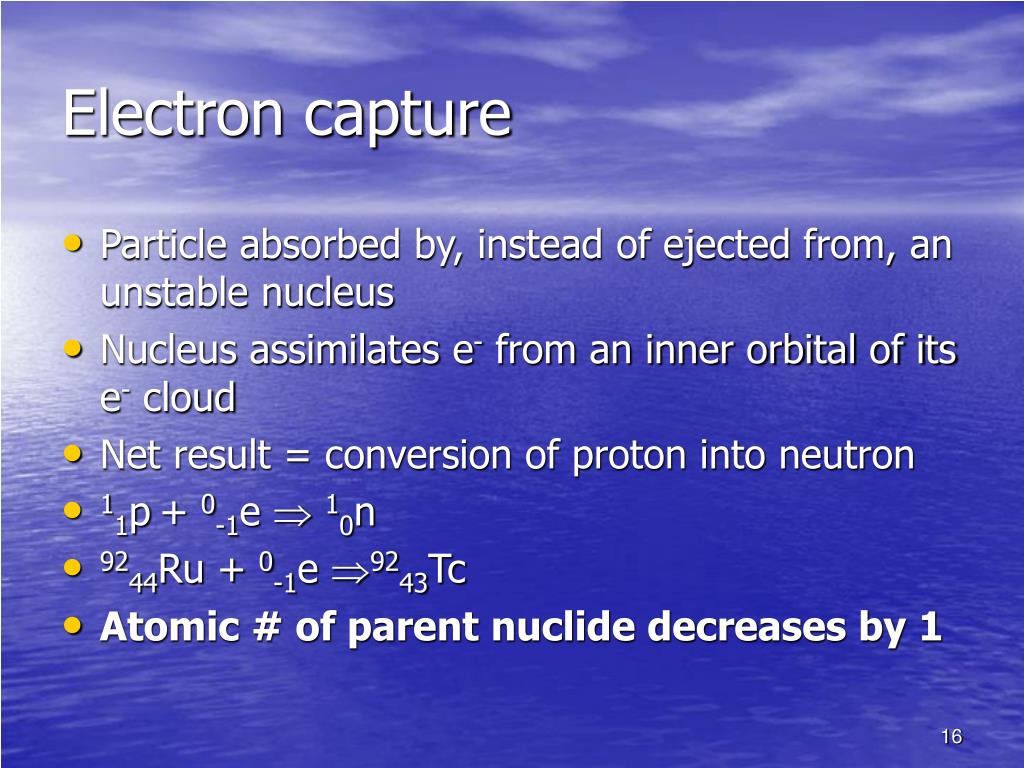
An atom with a gap in its electron structure rearranges itself, emitting X rays in the process or Auger’s electrons. Electrons are usually captured from the inner K layer, leaving ‘holes’ behind them. These events would go unnoticed if it were not for the restructuring that the nucleus and electron shells both undergo. The recoiling nucleus also barely moves, with the few microns that it covers being too small to be observed. Below this energy threshold, electron capture becomes the only process available to reduce an excess of protons.Įlecton captures often passes unseen, as the neutrino that carries away the released energy is impossible to detect. If the energy released in the decay is smaller than 511 keV, the emission of a positron (beta-plus decay) is not allowed. The creation of a positron requires 511 keV, the mass energy of the positron. However, electron capture is more economical in energy than positron emission, its competitor. The probability that an electron, even one belonging to the inner ‘K’ shell, would find itself inside the nucleus is very low indeed (for potassium 40, the volume of the nucleus is less than a billionth of the K layer volume). Whereas beta decay can occur spontaneously when energetically allowed, for an electron capture the weak forces require that the electron comes into close contact with a proton of the nucleus. Electron capture occurs much less frequently than the emission of a positron. Weak forces are behind positron emission and electron capture. This explains why electron capture is difficult and therefore rare.Įlectron capture : a difficult and rare process Even the innermost K-layer electrons are far from the very small volume of the nucleus where the weak forces responsible for the capture operate and transform the electron into a neutrino. Most of the electrons orbit the nucleus at distances large compared to the nucleus. The captured electron belongs to the group of electrons orbiting around the nucleus. Ordinary beta-minus decay has no competitor on Earth however to reduce an excess of neutrons, since the capture of positrons would occur in an world made of antimatter. Electron capture, along with beta-positive decay, is Nature’s way of guaranteeing that no nucleus becomes too proton-heavy. The capture of an electron has the same effect on a nucleus as the emission of a positron: one of its protons transforms into a neutron, diminishing the global electric charge of the nucleus by 1 unit. The electron capture trigger the emission of an invisible neutrino by the nucleus. The best-known example is of potassium 40 : 11% of the nuclei of that isotope of potassium present in our body decay by electronic capture. In both cases, practically all the enegy released is carried by light particles.Įlectron capture is a comparatively minor decay mode caused by the weak force. There is no such energy threshold in the case of electron capture (bottom). The emission of a positron and the capture of an electron are twin reactions both resulting in the diminution of the number of protons by 1 (from Z to Z-1) and the production of a neutrino.The positron observed in the final stage of the beta decay (top) is a new particle requiring the 0.511 MeV of its rest mass energy to be created.
It is this sensitivity that makes ECD the first choice for environmental measurements.Positron emission versus electron capture In comparison to a signal without sample compounds, the reduction in electron flow is proportional to the quantity of electrophile sample components.Įlectron Capture Detectors are up to 1000 times more sensitive than Flame Ionization Detectors and were the first detectors able to measure components at parts-per-billion (ppb) and parts-per-trillion (ppt) levels. If the GC effluent contains organic molecules with electronegative functional groups, such as halogens, phosphorous and nitro groups, electrons will be captured and the current will be reduced.
EXAMPLE OF ELECTRON CAPTURE FREE
By impact ionization, free slow-moving electrons are produced, which generate a measurable and steady current. Fast beta particles generated by the radioactive source collide with the molecules of the carrier or make-up gas. With an electron capture detector, a beta emitter such as radioactive tritium or 63Ni is used to ionize the carrier gas. Non dispersive infrared (ND-IR) spectroscopyĮmission testing for on-road and off-road vehicles and machineryĮlectron capture detectors (ECD) are typically used in environmental testing for detecting PCB’s, organochlorine pesticides, herbicides and various halogenated hydrocarbons. High performance liquid chromatography (HPLC)įourier transform infrared (FT - IR) spectroscopy


 0 kommentar(er)
0 kommentar(er)
